Surface preparation is one of the most important factors affecting the quality of the product after paint application.
Before the paint application process, it is the cleaning of grease, welding dirt and similar impurities on the surface of the material arising from the production by applying solvent, acidic and alkaline chemicals from the material surface.
The surface preparation process does not have a protective feature. It cleans the material and makes it suitable for the paint to adhere to the surface. Surface treatment is applied to increase the corrosion resistance of all paint coatings.
As a result of surface treatment, corrosion resistance and adhesion of paint to the surface increase. Corrosion and other problems in the coating may result from improper application of the surface treatment process. Surface treatment is usually more important than the final coating. Generally, two types of surface preparation methods are used. The surface preparation method can be selected according to material structure and capacity.
Generally, two types of surface preparation methods are used. The surface preparation method can be selected according to material structure and capacity.
- Dipping type surface preparation
- Spray type surface preparation
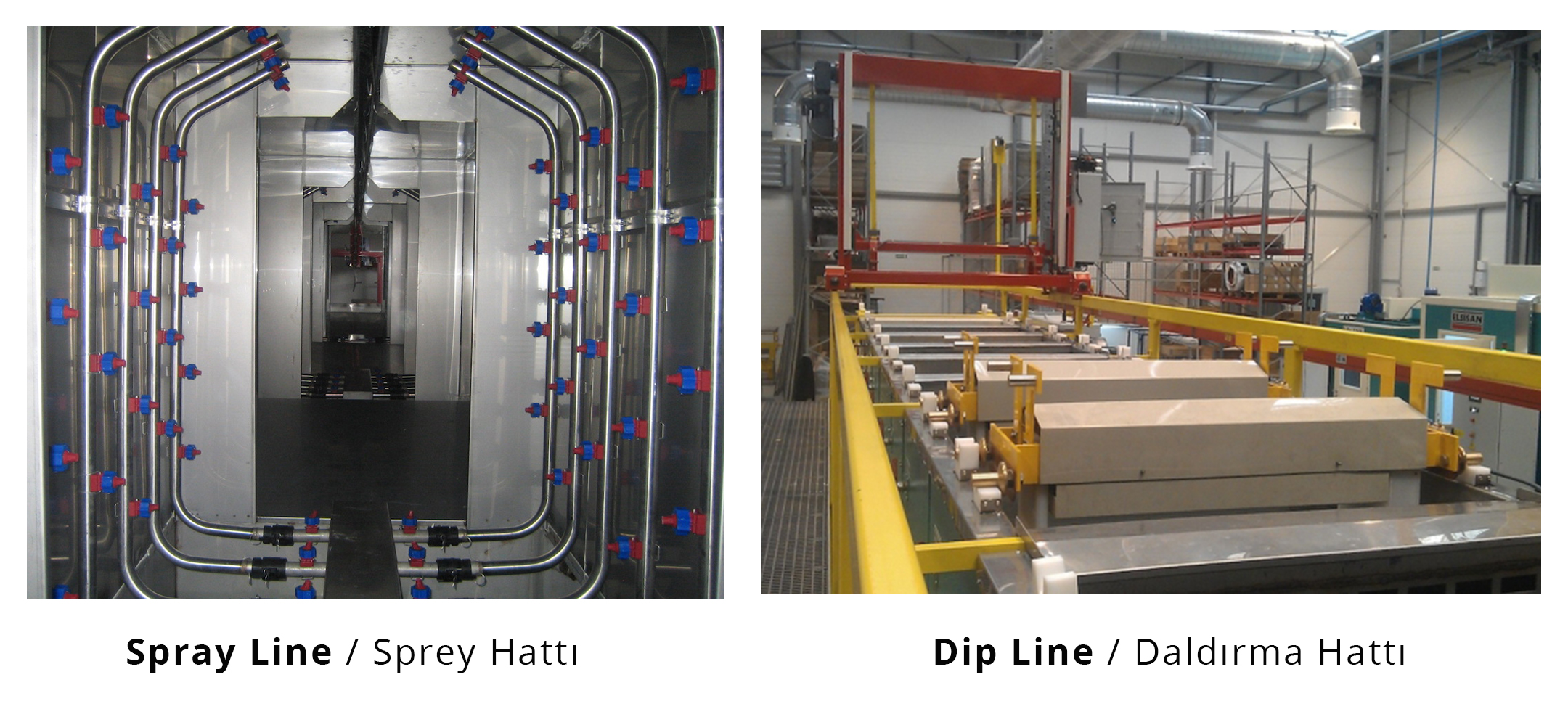
The factors that affect the surface preparation process sequence and selection are mainly,
- Quality and type of metal
- Condition of the surface: pollution rate and types of pollution that need to be cleaned from the surface
- Finished product, using areas and the level of protection required
- Economic and environmental factors.
The most basic chemical cleaning solutions, depending on the above factors,
- Iron Phosphate
- Zinc Phosphate
- Nano Coating
A. Iron Phosphate
It is applied to surfaces that do not require high corrosion resistance. For this reason, it is preferred in the coating of products used in closed environments that will be exposed to less corrosion. Since the number of baths is less than the zinc phosphate coating, the operating and investment costs are lower. Spray pressure should be chosen between 1.5 – 2 bar in spray pre-treatment lines. Degreasing and iron phosphate baths are between 60°C ±5°C. The pH value of the iron phosphate bath should be monitored so that it remains between 5 ± 1. Values to be followed in the relevant process, temperature, pH, conductivity, pump pressure, nozzles and time.
Basically the process sequence,
- Degreasing
- Rinsing
- Iron Phosphate
- Rinsing
- Passivation
- DI Rinsing
- Drying oven
B. Zinc Phosphate
It is used on surfaces that require high corrosion resistance. It is a type of phosphating commonly used in the automotive, electronics and white goods industries. Spray pressure should be chosen between 1.5 – 2 bar in spray washing lines. The degreasing baths should be at 60°C ±5°C and the zinc phosphate bath at 50°C ±5°C. The values to be followed in the relevant process are temperature, pH, conductivity, pump pressure, nozzles and time.
Basically process sequencing:
- Degreasing
- Degreasing
- Rinsing
- Activation
- Zinc Phosphate
- Rinsing
- Passivation
- DI Rinsing
- Drying Oven
C. Bonderite (Nano Coating)
There is almost no waste compared to phosphate coating applications. While water usage is reduced in this type of coating, it reduces the COD and BOD load to zero as it does not contain heavy metals. Since there is no need for heating as in zinc and iron phosphate baths, energy costs are significantly reduced. Cleaning time and maintenance costs are also reduced as there is no clogging in nozzles, filters and heat exchangers as there is no waste. Nano coating process time is shorter than phosphate coatings. In corrosion tests, it is seen that iron phosphate and zinc phosphate provide corrosion resistance.
The values to be followed in the relevant process are temperature, pH, conductivity, pump pressure, nozzles and time.
Basically process sequencing:
- Degreasing
- Rinsing
- DI Rinsing
- Nano Coating
- Rinsing
- DI Rinsing
- Drying Oven
Degreasing Types
1. Degreasing
All metal parts contain grease on their surface. The purpose of the grease on the metal part’s surface is to protect it from corrosion until the parts come to the process phase. The degreasing process is generally implement in the first phase of pre-treatment. The degreased part becomes ready for rust removal and phosphate application. Applying these processes to the metal with a greasy surface does not give healthy results, and the main problem arises after the paint is made. Color differences can cause yellowing and lightening of the paint. The degreasing process can be done alkaline, acidic or neutral. The type of metal, the way of application, the type of grease determine the properties of the chemical to be used. In a correct degreasing, it is desirable to have the properties of heating the surface, penetrating deep, removing the grease, breaking the grease, emulsifying and rinsing easily. Degreasing chemicals are used by dipping, spraying and wiping methods. The degreasing process is carried out by saponification of vegetable or animal oils and emulsification of mineral oils. It is also important that the metal does not darken and does not wear heavily in degreasing baths.
Bath control parameters to be considered are:
- Temperature
- Concentration
- Total Alkalinity
- Time
a. Alkaline Degreasing
The alkaline degreasing process is carried out in hot and alkaline baths. Alkaline degreasing chemicals are used in concentrations such as 1 – 15%. This change in concentration varies due to the difference in the amount of grease as well as the design of the line as dipping or spraying. The pressure effect in the spray baths will minimize the chemical percentage. Density in the amount of grease will increase the chemical ratio. The pH of the bath is monitored between 10 – 13. Temperature is the most important parameter to be followed in alkaline degreasing. The operating temperature varies between 50-95°C. The action that can be taken at low temperatures is to extend the time by increasing the concentration. The degreasing of the materials entering the degreasing bath can be taken between 5 and 20 minutes.
b. Acidic Degreasing
The Acidic Degreasing process is generally used for parts with rust on the metal surface. As grease is removed from the metal surface, rust is also cleaned. This type of degreasing works in an acidic environment. It certainly contains acids, (phosphoric, hydrochloric, sulfuric etc.) wetting agents and sometimes inhibitors. The pH of the Acidic Degreasing bath is between 1 and 2. Although the bath temperature can be used at ambient temperature, using it at high temperatures will increase the degreasing capacity and the reaction rate. Chemical concentration can be used between 5 – 50%. Metal surfaces with deep rust are cleaned in high concentration baths. Bathroom material must be made of stainless or plastic material with high acid resistance. In addition, after the acidic degreasing bath, the metal surface becomes susceptible to corrosion. For this reason, the part must be taken to the next process bath immediately.
c. Neutral Degreasing
Neutral Degreasing chemicals are generally used in spray surface treatment lines. It is also used as a degreasing agent in iron phosphate baths. Since the pH range is 6 – 8, its effect is very less compared to acidic and alkaline degreasing. The most common use is in the form of degreasing in iron phosphate lines. Generally, spray iron phosphate chemicals contain degreasers, but when the bath concentration begins to decrease, neutral degreasing agents are added. It is preferred because it is used in low concentrations such as 1-2%, as well as being treated with iron phosphate in the same bath. Working at temperatures between 50 – 55°C also provides a separate advantage.
Naim UZEL
Project Engineer
Project Department
Elsisan A.Ş

 TR
TR EN
EN